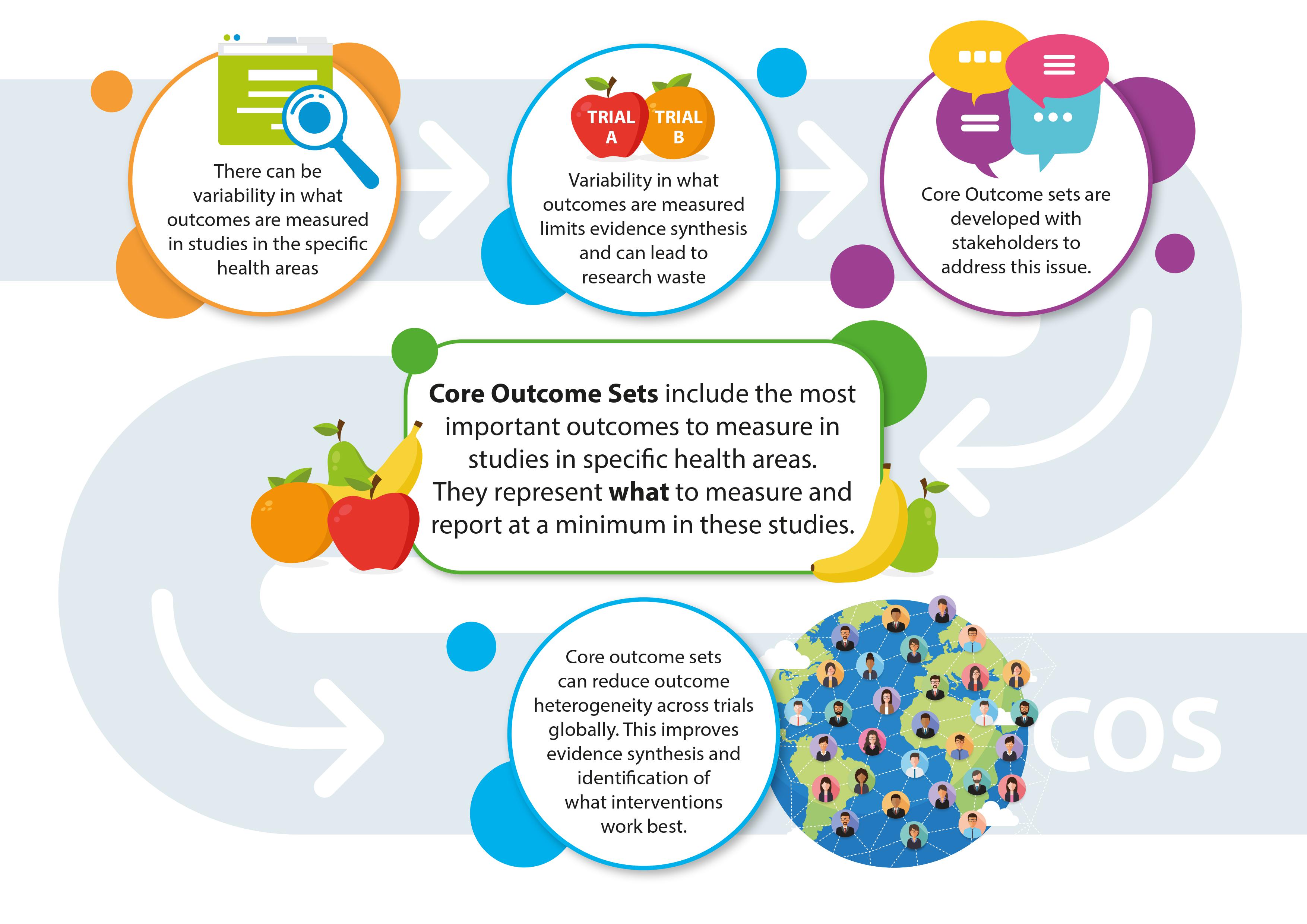
Core outcome sets (COS)
Core outcome sets (COS) are standardised agreed upon sets of outcomes to be measured and reported in trials for specific health conditions or areas. COS are developed through a systematic process that includes incorporating input from key stakeholders, including patients, clinicians, and policymakers, to ensure that research outcomes are relevant and applicable to real-world contexts and stakeholder needs. The main steps in the development of a COS include: 1) identifying what outcomes have been measured previously (e.g., via a systematic review), 2) prioritising outcomes (via a Delphi survey process), and 3) reaching consensus on the final core outcome set (via a consensus meeting). Developing and using COS can reduce outcome heterogeneity in trials, thereby improving evidence synthesis and reducing research waste. Use of COS can also help to reduce selective reporting of outcomes, and increase the relevance of trial results to stakeholders. Further details and resources about COS can be found at the Core Outcome Measures Effectiveness Trials (COMET) Initiative website: https://www.comet-initiative.org/

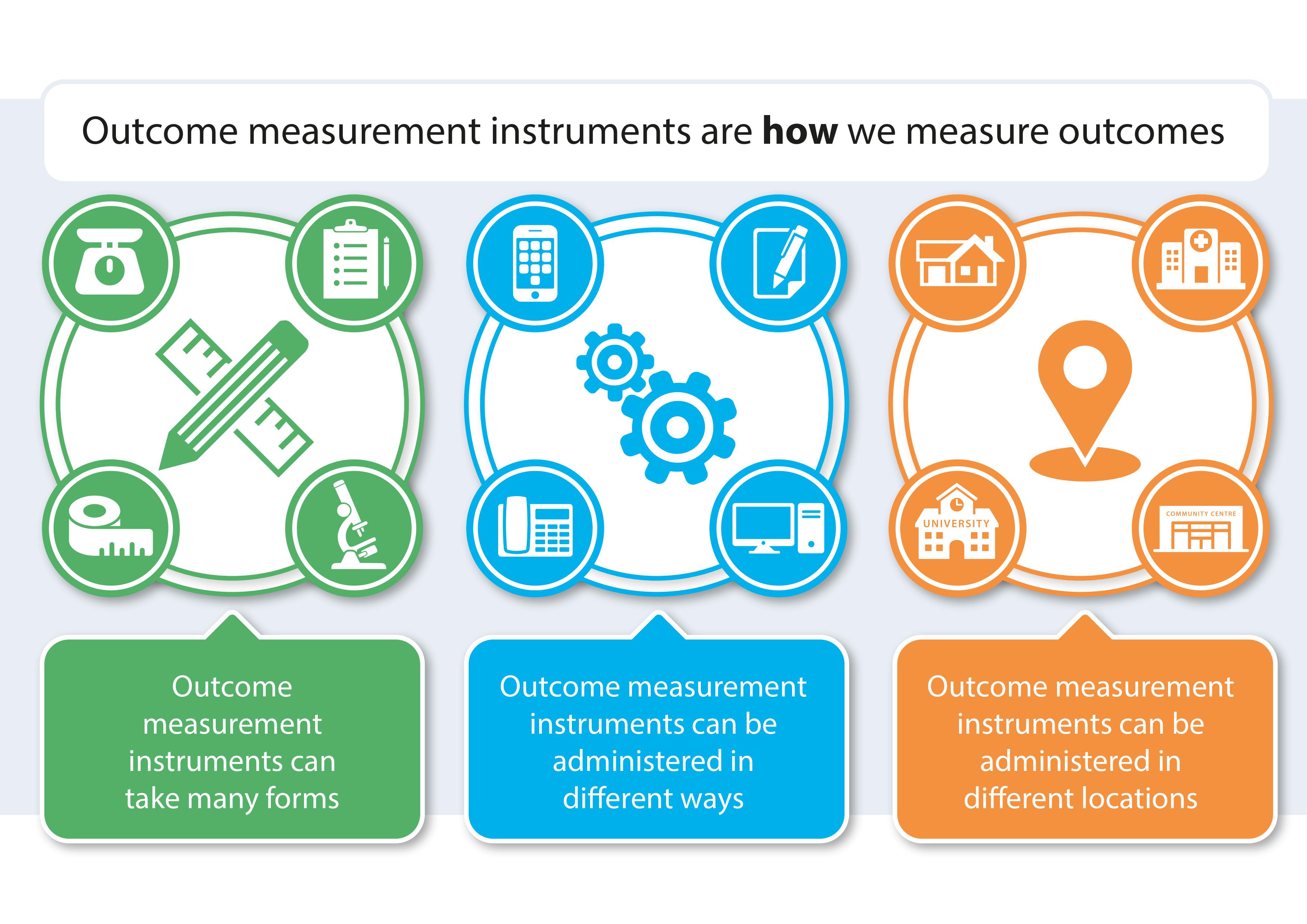
Outcome Measurement Instruments (OMIs)
The term outcome measurement instrument (OMI) refers to how an outcome is measured. Examples of OMIs include a questionnaire, single questions/items in a survey, diary records, observational methods, or clinical measurements such as weighing scales or callipers. OMIs can be used by researchers and healthcare professionals to evaluate intervention effectiveness, disease progression, and overall health outcomes. Choosing appropriate, feasible, reliable, and valid OMIs enables comprehensiveness, accuracy, and validity of data collection, thus facilitating informed decision-making, and evidence-based practices. Standardised sets of OMIs include OMIs that have been identified as reliable and appropriate/feasible by stakeholders. Using standardised OMIs can ensure uniformity in how outcomes are assessed, enhancing the comparability of research findings. Development of a standardised set of OMIs can be informed by guidance from the COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) Initiative, which includes identifying existing measurements, evaluating measurement properties of OMIs, engaging with stakeholders, and making recommendations. Further information from the COSMIN Initiative can be found here: https://www.cosmin.nl/.